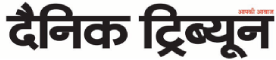
Safety needed: Quick approval of vaccines for coronavirus has created mistrust. Reuters
KK Talwar
Former Director, PGIMER
The year 2020 shall be remembered as the year of Covid-19, a viral disease which affected and shook the whole world in less than three months. The world still does not know exactly how this virus originated in the city of Wuhan in China in December 2019; the highly contagious nature of the virus was also not realised by the local experts and the WHO, and thus, effective preventive steps like restrictions on travel were not taken initially to control its spread globally.
This fast-spreading viral disease diversely affected society all over and was declared a pandemic by the WHO in March 2020. Though most of the patients are mildly symptomatic,
15-20% are moderately to severely symptomatic. Many precious lives were prematurely lost as there was no definite treatment to cure this disease. The helplessness of the medical community understandably terrified the people.
Realising the lack of a medical solution, lockdowns were imposed in most countries to control its spread. This certainly helped to control the initial spread and gave time to strengthen hospital care facilities to face this unusual health crisis. But of course, the lockdowns and other restrictions caused serious collateral damage to the economy and the social system. Many lost their jobs and livelihood. The photographs of migrants travelling hundreds of kilometres on foot to their far-off homes because of the loss of jobs still send shivers down the spine. The subsequent restrictions imposed for control of spread of this virus still continue to affect the social and business activities of the country. The mental agony of those who contracted this virus and their families is unimaginable.
Since then, the medical community has worked tirelessly to understand this disease and find medical solutions. But all these efforts until now have failed to find the right medicine to prevent or effectively treat this disease. Of course, growing understanding of the pathogenesis and course of the disease has helped to adopt some therapeutic approaches which have saved some patients in the absence of any effective anti-viral drug.
Not surprisingly, the innovative and heroic efforts of the medical researchers to find new effective drugs and vaccines have produced results. A few effective vaccines, based on their safety and efficacy studies, have been granted emergency use authorisation (EUA) approval. Two vaccines — from Pfizer and Moderna — based on the new mRNA platform, have been approved in the UK, US, and other parts of Europe. The Oxford-AstraZeneca vaccine has also been approved in the US and the UK. In India, the Oxford and the Bharat Biotech vaccines have also been approved. These vaccines have presently been approved for EUA in view of the limited experience of their long-term safety and effectiveness.
The Russian and Chinese vaccines have also given approval in their respective countries. Many more vaccines are under study and only time will tell which vaccine proves to be ideal for this disease. All the approved vaccines need two doses, varying from 14 to 28 days for the second one. Normally, it takes nearly a decade for the approval of a vaccine, but all this has presently happened in 10 months, highly creditable for medical scientists. This ensures the biggest hope of 2021, to control this pandemic. But this unprecedented speed to approve the vaccine has generated mistrust regarding the safety and long-term immunity protection. The initial data does ensure safety, but how long the immunity passport lasts, we shall learn only with time. The only precaution is to avoid certain categories like the young (below 18 years), pregnant women and those with an allergic background. The general instruction to observe a vaccinated person for 30 minutes in the hospital is to handle such rare allergic reaction.
Though the vaccines are expected to control the spread of the disease, recent observations detecting significant mutations (UK and South African variants) in the virus have raised concern about the effectiveness of the available vaccines for the mutant strains. This has caused immense distress at a time when the availability of vaccine has brought comfort and hope to society. Still, it has been emphasised that these mutations do not affect the efficacy of the presently approved vaccines. The variant strains have been shown to be more contagious, but it is not clear if these are more pathogenic. Needless to say, we need to be cautious about any future pathogenic mutations.
Efforts to produce effective antiviral drugs are on. New anti-viral drugs like Umifenovir and Sofosbuvir, which are going through phase 2 trials, appear promising. IL6 receptor antagonist viz Sarilumab and Tocilizumab are being evaluated and found promising in severe Covid-19 cases (in Cytokine storm). Similarly, JAK inhibitors like Baricitinib and Ruxolitinib are also being evaluated and have shown promising results in severe Covid cases. Another approach of infusing a monoclonal antibody cocktail (neutralising antibody combination) has also been shown to control deterioration from moderate to severe state. Their phase III trials are still on. Thus, we hope to have an effective antiviral drug, IL 6 inhibitors and monoclonal antibody, to treat these patients.
Thus, 2021 offers new hope and optimism over having an effective drug in the near future in addition to a vaccine to control this pandemic.
Till the vaccines are freely available and over 60% of the population is inoculated, which will result in herd immunity, we need to be cautious and take preventive steps. The protective measures for Covid-19, including the use of a mask, social distancing, hand
hygiene and avoiding closed and crowded places, need to be adopted as our social vaccine to save ourselves from the coronavirus.
Join Whatsapp Channel of The Tribune for latest updates.